Entropy production in biology
Heat is essential for life. In living organisms, generating mechanical and chemical work to accomplish specific tasks inevitably leads to energy dissipation in the degraded form of heat—the most fundamental signature of life. Most metabolic reactions in our body are exothermic and generate heat, which is used to keep the homeostatic conditions in our body. From a thermodynamic point of view, work is the highest form of energy, whereas heat is the most degraded one: while work can be fully converted into heat, the opposite is not true. Interestingly, many molecular machines in our body, such as the ATP-synthase in the respiratory chain, can operate quasi-reversibly near 100% efficiency. Yet, in all cases, heat is produced in large amounts inside cells, and the balance between heat, or entropy production, and the rest of the valuable things life produces, coined as negentropy by Schrödinger, makes up the energy balance of life.
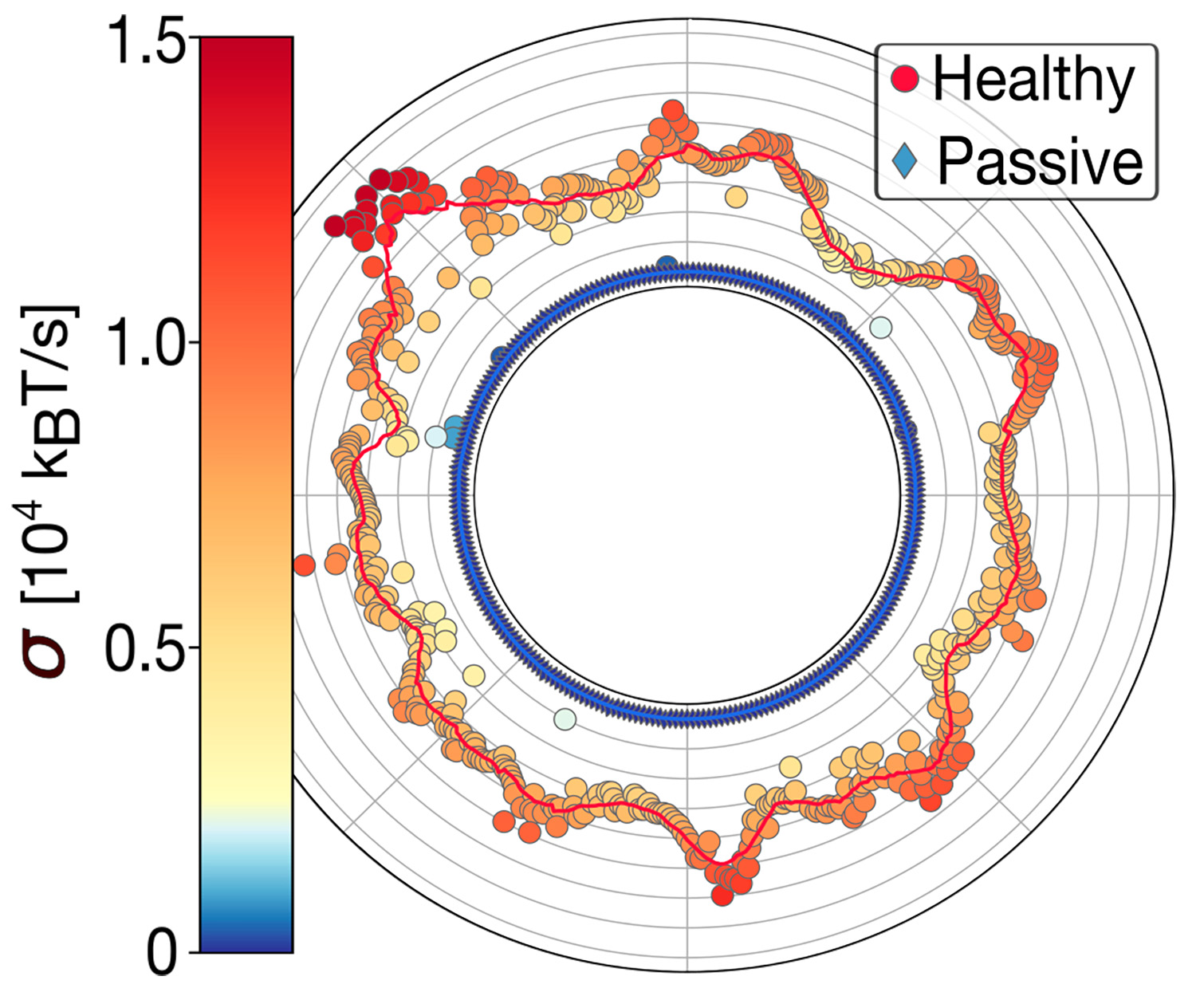
Despite major progress in processing large biological data sets across all omics layers, we still lack a clear understanding of measuring heat generation at the nanoscale, known as the entropy production rate σ. We have recently introduced a variance sum rule (VSR) for measuring σ with attowatt sensitivity (1aW = 10-18 Watts) from the flickering motion of a microscopic probe sensitive to the local environment [1]. The VSR has paved the way to heatomics, the science of heat for life. The entropy production reflects the activity of metabolic reactions, and it should not be confused with the effective temperatures measured in nanothermometry, which quantify violations of the fluctuation-dissipation theorem and are indirectly related to σ [2]. The VSR has led to the first heat map of human red blood cells [1], known for their remarkable deformability properties triggered by the glycolytic activity [3]. The variance sum rule combines experiments and modelling [4] and is a fundamental resource for heatomics and the energetics of life. Currently, we are extending our measurements to hidden degrees of freedom, which find applications in enzymatic reactions, from oxidoreductases to hydrolases and ATPases, e.g., molecular motors translocating along a substrate [5,6].
Finally, we would like to mention evolutionary ensembles, a remarkable category of systems that transduce energy from the environment into diversification, or information production, across evolutionary timescales. Inspired by fluctuation theorems in stochastic thermodynamics, we have conducted theoretical and experimental studies to quantify the information content of a mutational ensemble of DNA hairpins. The right combination of theory and experiments will be essential to uncovering physical principles of evolution based on measurements of energy and information.
[1] I. Di Terlizzi, M. Gironella, D. Herraez-Aguilar, T. Betz, F. Monroy, M. Baiesi, F. Ritort, Variance Sum Rule for Entropy Production, Science 383 (2024) 971-976
[2] E. Dieterich, J. Camunas-Soler, M. Ribezzi-Crivellari, U. Seifert, and F. Ritort, Single-molecule measurement of the effective temperature in non-equilibrium steady states, Nature Physics, 11 (2015) 971–977
[3] M. Gironella-Torrent, G. Bergamaschi, R. Sorkin, G. Wuite, F. Ritort, Viscoleastic phenotyping of red blood cells, Biophysical Journal 123 (7), 770-781 (2024)
[4] I. Di Terlizzi, M. Baiesi, F. Ritort, Variance Sum Rule: proofs and solvable models, New Journal of Physics, 26 063013 (2024)
[5] M. Manosas, S. K. Perumal, P. Bianco, F. Ritort, S. J. Benkovic, V. Croquette, RecG and UvsW catalyse robust DNA rewinding critical for stalled DNA replication fork rescue, Nature Communications, 4 (2013) 2368
[6] V. Rodriguez-Franco, M. Manosas and F. Ritort, Controlled transport by molecular machines: exploring biological motors and their physics, Europhysics News 55, 20-23 (2024)